Igniting Therapeutic Innovation with Molecular Intelligence
We unite AI, expansive chemical space, and
biological insight to deliver smarter molecules,
richer data, and novel opportunities for
breakthrough therapies.
Use our contact form or email us directly to schedule an introductory call. We’re ready to learn about your goals and explore how we can support your discovery efforts.
Your Project, Powered by Our Chemistry
- ChemoVista™ for 8M+ in-stock compounds
- VirtuSynthium™ for 10¹⁶ virtual synthesis-ready molecules
- OmniPeptide Nexus™ for limitless peptide design
- MacroCyclePro™ for macrocycle discovery
- Receptor360™ for protein-centric design across 8,500+ targets
REAXENSE™ is a powerful AI-enabled platform built to unlock novel chemical space for therapeutic innovation. By combining vast in-stock and virtual compound libraries, peptide and macrocycle design tools, and dynamic protein characterization for 8,800+ targets, REAXENSE helps researchers discover, optimize, and synthesize smarter molecules—faster and more precisely than ever before.
REAXENSE dedicated chemical spaces
ChemoVista™
The AI-Driven Chemical Space for
Drug Discovery
Key features:
A vast chemical repository optimized for hit identification, lead optimization, and high-throughput screening.
Seamlessly integrated with Receptor.AI, ChemoVista enables AI-powered virtual screening, SAR modeling, and data-driven compound selection. The space is curated for:
- Diverse molecular architectures (T mean < 0.35, broad scaffold coverage, sp²/sp³ balance, ~2,500,000 of unique Murcko scaffolds)
- Optimized physicochemical properties (MW, cLogP, HBD, HBA, TPSA, RB) – 90% of ChemoVista are drug-like (Ro5-compliant) compounds
- ADMET-compatible profiles (MW, cLogP, HBD, HBA, TPSA, RB) – 90% of ChemoVista are drug-like (Ro5-compliant) compounds
- Commercial Availability: 100% in-stock and competitive pricing for seamless procurement.
Plated compounds, custom
assay plates, and bulk
supply options ready for
immediate worldwide
shipment.
Every compound undergoes
HPLC, NMR, MS, and
stability testing,
ensuring reproducibility
and high-quality data.
Purity: min 90%, max
99%.
Compounds provided in
standardized formats
(SDF, SMILES, InChI,
etc.), ensuring
compatibility with
computational pipelines
and AI-driven
screening.
Single-package
shipments,
temperature-controlled
handling for DMSO
solutions, and free
customs clearance for US
& EU customers.
VirtuSynthium™
AI-Powered Virtual Chemical Space
for Next-Generation Drug
Discovery
Key features:
VirtuSynthium provides
access to a virtual
library of 10¹⁶
synthetically accessible
molecules—the largest
AI-navigable chemical
space available —
constructed through the
combinatorial
application of over
1,000,000 validated
reagents and building
blocks using diverse,
reaction-aware design
protocols for dynamic
and scalable molecular
generation.
Advanced machine
learning, predictive
ADMET modeling, and
AI-guided virtual
screening enable precise
compound selection for
high-impact drug
discovery.
Unlike static enumerated
libraries, VirtuSynthium
incorporates real-time
synthetic feasibility
assessment using over
1,000 validated reaction
pathways and qualified
building blocks,
ensuring that each
proposed compound is
practically accessible
and can be synthesized
and delivered within
industry-standard lead
times, starting from as
little as 4 weeks.
| Category | Factor | Description | Typical Range / Example |
|---|---|---|---|
| Combinatorial | Building blocks (BBs) | Input molecules (e.g., amines, acids, aldehydes) | 100 – 100,000 per class |
| Reaction steps | Sequential synthetic steps (1–4 typical) | 1–4 steps; exponential growth (e.g., 1k³ = 10⁹) | |
| Substitution positions | Number of R-group attachment points on scaffolds | 2–6 positions | |
| Substituent diversity | Number of substituents per position | 10–10,000 | |
| Scaffold cores | Distinct molecular cores or frameworks | 1,000 – 1,000,000 | |
| Structural | Stereoisomers | 2ⁿ where n = number of stereocenters | Up to 2⁸ = 256 |
| Tautomers / Protonation states | Variants of same molecule | 1–5 per structure | |
| Tanimoto similarity (diversity threshold) | Measures uniqueness in fingerprint space | Typical cutoff: 0.75 – 0.85 | |
| Ring systems | Distinct cyclic frameworks | 100,000+ possible | |
| Physicochemical | Molecular weight | Size filter to maintain drug-/lead-/fragment-likeness | <350 (fragment), <500 (drug-like), >500 (bRo5) |
| cLogP / cLogD | Lipophilicity filter | -2 to 5 (drug-like) | |
| H-bond donors / acceptors | Structural filter for oral bioavailability | ≤5 donors, ≤10 acceptors | |
| TPSA / Rotatable bonds | Controls permeability and flexibility | TPSA < 140 Ų; ≤10 rotatable bonds | |
| Rule-based filters (PAINS, REOS, etc.) | Remove unwanted motifs | Binary (pass/fail per structure) | |
| Computational | Storage and enumeration | Raw memory or disk space for storing molecules | 1 trillion SMILES ≈ ~100 TB uncompressed |
| Fingerprint indexing | Efficient representation (e.g., ECFP4, MHFP) | 256–2048 bits per molecule | |
| On-demand vs pre-enumerated | Whether molecules are pre-built or generated live | On-the-fly preferred at >10⁹ scale | |
| Search speed (per query) | Speed for similarity or substructure search | <1 second for 10⁹ molecules using optimized tools | |
| Synthetic Feasibility | Reaction rules (RECAP, BRICS, SMARTS-defined) | Determines synthetic routes and bond types allowed | 50–1,000 generic reactions |
| Commercial & Tangible BB availability | Whether BBs are purchasable | 100K – 10M | |
| Synthetic accessibility score (SAS) | Machine-learned or rule-based score for ease of synthesis | Scale: 1 (easy) – 10 (hard) | |
| Application-Driven | Target class constraints | Filters based on target type (e.g., kinase hinge binders, GPCR-like) | Custom pharmacophore-based rules |
| 3D shape similarity | Compared to known ligands (e.g., FTrees similarity) | Threshold: 0.80 – 0.95 | |
| Patent novelty / FTO filters | Exclude structures too similar to known compounds | Similarity or substructure filters (Tanimoto < 0.7 to known space) |
SAS < 7 ensures that
AI-generated molecules
are not only innovative
but also synthetically
actionable, streamlining
the path from design to
synthesis and
ultimately, to clinical
relevance.
Directly embedded in the
REAXENSE platform,
facilitating hit
identification, lead
optimization, and
structure-based drug
design with IP-free
exploration.
Balances Rule of Five
(Ro5) and Beyond Ro5
(bRo5) compounds,
including macrocycles,
peptidomimetics, and
PROTAC-like structures,
for tackling previously
undruggable targets.
VirtuSynthium
streamlines hit-to-lead
workflows by integrating
AI-driven compound
prioritization with
synthetic feasibility
modeling, significantly
reducing development
timelines, cost burdens,
and attrition rates —
thereby enabling
researchers to
efficiently navigate and
exploit untapped regions
of chemical space.
OmniPeptide Nexus™
AI-Powered Peptide Design
Key features:
- Cyclic peptides for enhanced metabolic stability and membrane permeability.
- Branched and dendritic architectures for multivalent binding or scaffold rigidity.
- Stapled peptides using hydrocarbon linkers to stabilize α-helical structures and modulate protein–protein interactions.
- Chemically modified peptides, such as N-methylated backbones, D-amino acid incorporation, and unnatural amino acid substitutions to resist proteolysis and enhance selectivity.
| Category | Details |
|---|---|
| Peptide Length | Short: 2–10 AA; Medium: 10–50 AA; Long: 50+ AA |
| Sequence Diversity | 20⁵ = 3.2×10⁶ (5-mer); 20¹⁰ = 1.02×10¹³ (10-mer) |
| Amino Acid Options | 20 standard AA; 500+ non-standard AA |
| Structural Constraints | α-helix: ~3.6 AA/turn, 5.4 Å pitch; β-strand: ~3.5 Å/AA; Cyclic turn: 7–10 AA |
| Stability Factors | Half-life: minutes–hours; enhanced by D-AAs, N-methylation, cyclization |
| Binding Strength | Kd: low μM to nM; H-bonds: ~2–5 kcal/mol; Salt bridges: ~1–3 kcal/mol |
| Binding Strength | SPPS up to ~50 AA; Coupling efficiency: 95–99% per step |
Each sequence is contextually generated based on target-specific information, including predicted or experimentally resolved binding pockets, known epitope regions, or disease-relevant motifs.
Receptor.AI’s platform
integrates LLM-based
literature mining,
protein-ligand
co-evolution analysis,
and sequence-to-function
prediction algorithms,
enabling the rapid
generation of peptide
libraries that are
structurally diverse,
IP-novel, and
therapeutically
actionable.
Use Receptor.AI’s
ensemble-based
conformational analysis
to model dynamic peptide
structures. Integrate
real-time 3D
visualization and
AI-guided SAR tools for
affinity, selectivity,
and stability
optimization.
Predict and rank
peptide-target
interactions using
Receptor.AI’s
proprietary deep
learning models for
binding affinity, ADMET,
and immunogenicity. Map
interactions with
protein–protein
interfaces and
allosteric sites.
Enhance pharmacokinetics
via AI-driven prediction
of cyclization,
PEGylation, lipidation,
or D-amino acid
substitutions—guided by
Receptor.AI’s molecular
dynamics and de novo
design engines.
Transition from design
to synthesis using
automated planning and
virtual reagent matching
within the REAXENSE
ecosystem. Deliver
peptides at scale, from
research-grade to
GMP-compliant
formats.
Ideal for oncology, infectious diseases, immunotherapy, and targeting “undruggable” PPIs. OmniPeptide Nexus empowers faster, smarter peptide drug discovery—fully embedded in Receptor.AI’s discovery pipeline.
MacroCyclePro™
AI-Driven Macrocyclic Drug Discovery
Platform
Key features:
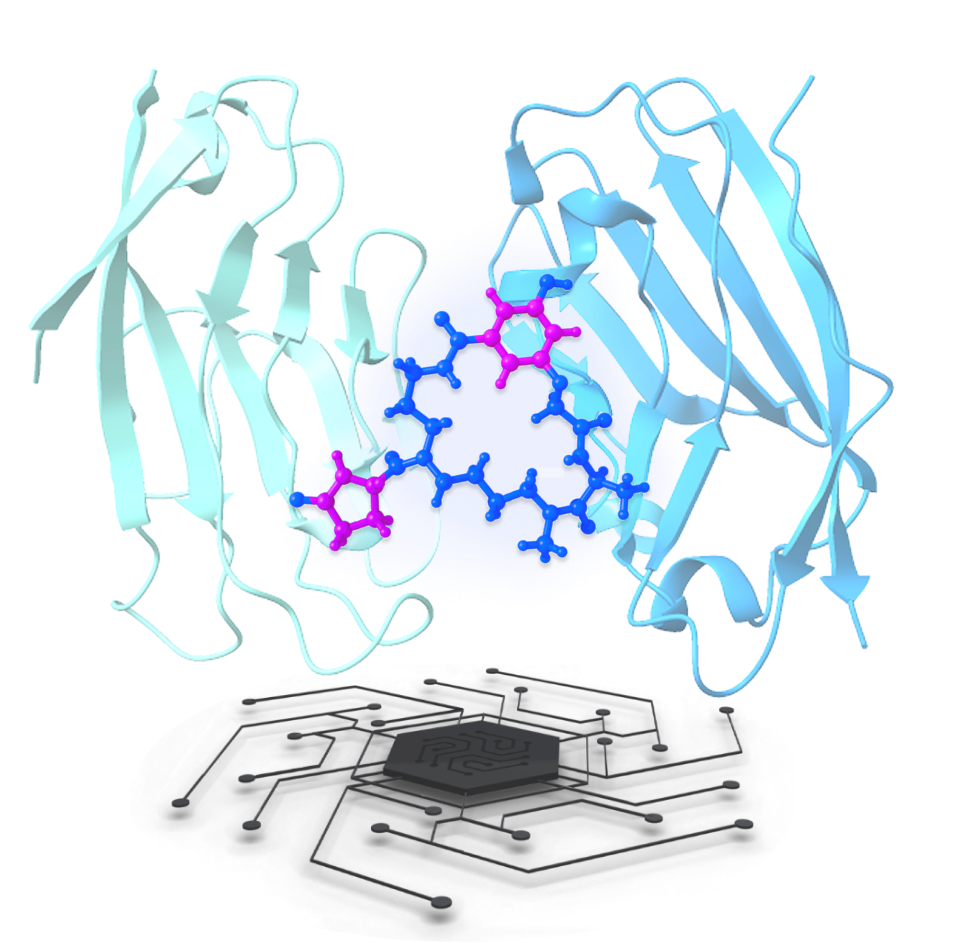
Utilizes
transformer-based models
and generative
algorithms (e.g.,
ProtGPT, LSTM, GANs) to
design chemically and
structurally diverse
macrocycles—including
cyclic peptides, bridged
scaffolds, and
peptidomimetics—optimized
for target engagement,
stability, and
synthesis.
| Category | Parameter | Typical Values / Notes |
|---|---|---|
| Ring Size | Small Macrocycles | 8–12 atoms (strained, less common) |
| Medium Macrocycles | 12–20 atoms (optimal for bioactivity) | |
| Large Macrocycles | >20 atoms (flexible, e.g. antibiotics) | |
| Preferred Peptide Macrocycles | 14–20 atoms | |
| Cyclization Energy Cost | ~5–15 kcal/mol (entropy loss) | |
| Conformation | Average Diameter | 5–15 Å |
| Intramolecular H-bond Contribution | ~2–5 kcal/mol each | |
| Rotatable Bonds | ≤8 improves oral bioavailability | |
| Bioactivity | Binding Affinity (Kd) | low μM to nM range |
| Molecular Weight | Can exceed 500 Da | |
| cLogP | Tolerated range: 2–6 | |
| Total Polar Surface Area (TPSA) | ≤140 Ų improves permeability | |
| Synthesis | Step Efficiency | 80–95% |
| Cyclization Concentration | mM–μM range | |
| Ring-Closing Metathesis (RCM) Success Rate | >70% for medium rings | |
| NRPS Cyclization Efficiency | ~95% | |
| Pharmacokinetics | Hydrogen Bond Donors | ≤4–5 improves permeability |
| Hydrogen Bond Acceptors | ≤10–12 recommended | |
| Plasma Half-Life | Typically 1–10 hours; extendable | |
| Protease Stability | Improved 10–100× by cyclization | |
| Stability Enhancers | N-methylation and d-amino acids enhance stability |
MacroCyclePro leverages
a multi-tiered DOS
strategy to construct
structurally diverse,
stereochemically rich
macrocyclic libraries.
The platform implements
both algorithmic
assembly routes and ring
distortion tactics to
expand scaffold
complexity and chemical
space coverage:
- Algorithmic Methods: Includes modular Build/Couple/Pair (B/C/P) workflows, iterative coupling (e.g., B/C/C/C/P), and cascade-based Initiate/Propagate/Terminate strategies. These approaches enable fine-grained control over ring size, topology, and functional group placement.
- Fragment-Based Domain Shuffling: Modular permutation of chemically orthogonal fragments enables scaffold recombination and novel topology generation—ideal for exploring ligand-efficient binding modes.
- Symmetric & Iterative Synthesis: Employs Two-Directional Synthesis for symmetrical precursor elaboration and Successive Ring Expansion (SuRE) protocols to iteratively enlarge macrocyclic rings via acylation and rearrangement.
- Ring Distortion Techniques: Starts from complex or natural-product-inspired frameworks and diversifies them via ring expansions, pericyclic transformations (e.g., Diels–Alder/retro-Diels–Alder), and scaffold rearrangement. These reactions enable rapid generation of topologically unique, conformationally preorganized macrocycles.
- Together, these strategies ensure broad coverage of skeletal, stereochemical, and appendage diversity, enabling access to macrocycles with properties tuned for bRo5 compatibility, synthetic feasibility, and challenging biological targets.
Integrated with
Receptor.AI, the
platform enables
real-time binding
affinity prediction,
conformational scoring,
and ADMET profiling.
Structural optimization
workflows refine hits
into high-quality leads
with enhanced
pharmacological
properties.
MacroCyclePro is
purpose-built to tackle
flat, groove-shaped, and
tunnel-like protein
interfaces—such as
protein–protein
interactions—through
preorganized,
conformationally
restricted scaffolds
capable of chameleonic
behavior and ternary
complex formation.
Focuses on chemically privileged macrocyclic space with rich stereochemistry, increased polar surface area, and high molecular weight—balancing permeability, solubility, and bioavailability via intra-molecular hydrogen bonding and scaffold engineering.
Every AI-designed
macrocycle is paired
with a validated,
cost-effective synthetic
route. MacroCyclePro
supports parallel
solid-phase peptide
synthesis, native
ligation, and custom
modifications with up to
99% purity in both
research and GMP-grade
formats.
Predictive AI tools
guide PEGylation,
lipidation, and
encapsulation into
nanoparticles or
microneedles to engineer
orally bioavailable and
injectable macrocyclic
drugs with sustained
release and improved
pharmacokinetics.
Contact us
Do you have a question?
Please complete our contact form and a member of our team will get in touch as soon as possible.




