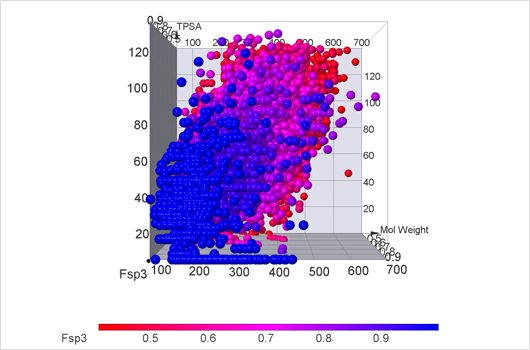
Fsp3 Compound Library
Scientific community studying medical chemicals has become increasingly aware of the value of the physical and structural properties of molecules. It is now known that the molecules which follow Lipinski's rule of five are more likely to appear biologically active. Other thresholds for properties were also proposed, for example, topological polar surface area and rotatable bonds number. Following the work of Frank Lovering et al. "Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success", which discovers saturation of chemical compounds as the measure of the likelihood of compounds to be biologically active, we created a library which comprises known thresholds of medical compounds and also contains highly saturated compounds.